LAB TESTS PROCEDURES AND RESULTS
Self-Cleaning
Lab Test Procedures and Results: Self-Cleaning The goal of this experiment was to determine if ambient light can work in conjunction with TiFusion™ coated apparel to clean apparel. A solution of approximately 10 mg/L of methyl orange was prepared in two 0.5 inch x 1.5 inch beakers. One beaker contained TiFusion™ coated t-shirt material and the other contained untreated t-shirt material. Both samples were placed in separate 50 mL beakers with 30mL of the prepared methyl orange solution. The samples submerged in the methyl orange solutions were left in the dark for 30 minutes to achieve adsorption equilibrium. After 30 minutes, both beakers were exposed to light for a period of three hours using a white light bulb lamp as the light source. A sample of the solution was measured using an ultraviolet-visible spectrophotometer to quantify how the concentration of methyl orange changes with time upon exposure to light with treated t-shirt material and with untreated t-shirt material. An absorbance reading was taken for each solution after every hour for three hours. The following results were observed.
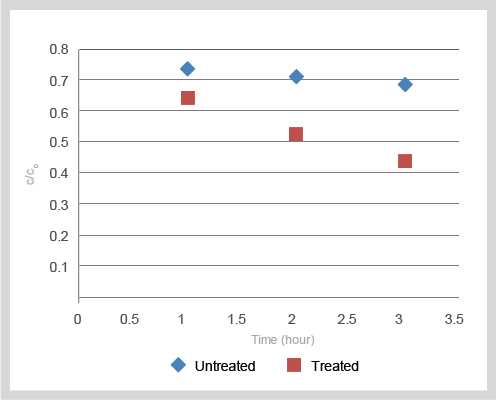 Figure 1: Effect of t-shirt material coating on self-cleaning
Figure 1: Effect of t-shirt material coating on self-cleaning
From Figure 1, the concentration of methyl orange in the treated and untreated samples decreases over time, but concentration of methyl orange decrease more dramatically for the treated sample. The result suggests that the TiFusion™ coating provides self-cleaning properties.
UV PROTECTION
To assess the amount of UV light blocked by each shirt we placed a small swatch of each t-shirt material placed underneath a UV lamp and on top of an ultraviolet light meter. Ten readings were obtained for both treated and untreated samples. Table 1 summarizes the result for this experiment.
Table 1: Result for UV Protection (amount of UV light blocked)
| UV reading | Intensity ( x 10 μs/cm3) | Percent UV light Absorbed |
|---|---|---|
| No material covering detector | 295.0 | 0.0 |
| Titanium oxide treated t-shirt | 6.8 | 97.7 |
| Untreated t-shirt | 12.1 | 96.1 |
From Table 1, the treated sample absorbed 1.6 % more UV light than the untreated sample.
INCREASE HEAT TRANSFER
We used the wet-bulb method to determine the increase in heat transfer. For this experiment, a sample of treated apparel was wrapped and tied around the bulb of the thermometer and wetted with water. The thermometer is then left in air until the sample is completely dried. Periodically the thermometer temperature is recorded. Changes in thermometer temperature correspond to evaporation of the water soaked into the t-shirt material. Energy is required to evaporate the water so the thermometer temperature will go down, reach equilibrium and stay at this temperature until all the water is evaporated. At this time the t-shirt will heat back up to room temperature. Figure 4 shows the set up for the experiment, and Table 2 shows the result.
Table 2: Result for increase heat transfer
| Time Min. | Temperature of Untreated (°C) | Temperature of Treated (°C) |
|---|---|---|
| 0 | 24 | 24 |
| 1 | 19 | 17 |
| 3 | 18 | 17 |
| 5 | 18 | 17 |
| 7 | 18 | 17 |
| 9 | 18 | 17 |
| 20 | 18 | 17 |
| 45 | 21 | 24 |
| 60 | 24 | 24 |

From Table 2, the temperature for a treated sample decreased to a lower temperature (17 °C) than the untreated (18 °C). The temperature of treated sample comes back to room temperature after 45 minutes, while it took the untreated sample 60 minutes. This suggests the TiFusion™ coating improves heat transfer. The rate of temperature change for the treated from minute 20 to 45 water 0.28 °C/min and for the treated from 20 to 60 minutes was 0.15 °C/min. A larger rate implies better heat transfer. Based on these results, TiFusion coated apparel dried 25% faster than uncoated apparel.
ODOR REDUCTION
The following experiment tested TiFusion′s ability to reduce odor causing Hydrogen Sulfide. This study consisted of four one-liter static Tedlar bags; control, treated (containing 3 inch square pieces of material), and untreated (containing a containing 3 inch square pieces of material). The bags were held at room temperature, ambient lighting and 50% relative humidity for the duration of the 24 hour experiment.
The control, treated and untreated chambers were exposed to approximately 1000ppbV of hydrogen sulfide and samples from each chamber were collected at five temporal points (0,1,4, 7 and 24 hours). All of the samples were analyzed according to ASTM D 5504-01 using a gas chromatograph equipped with a sulfur chemiluminescence detector (SCD). The following results were observed:
| Time | Control ppbV | Untreated ppbV | Treated ppbV |
|---|---|---|---|
| 0 | 925.8 | 823.4 | 942.1 |
| 1 hour | 972.1 | 817.0 | 794.0 |
| 4 hour | 916.5 | 696.8 | 437.2 |
| 7 hour | 898.6 | 620.5 | 173.0 |
| 24 hour | 847.9 | 330.6 | 8.6 |
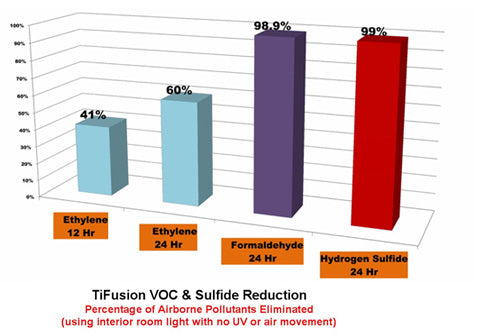
Results: over a 24 hour period treated material reduced levels of Hydrogen Sulfate from 942.1 ppbV to 8.6 ppbV. Untreated material had a reduction of 823.4 ppbV to 330.6 ppbV. Treated material had a 99% reduction rate compared to a natural reduction rate of 59%.
Reduction rates of odor causing compounds (including: Ethylene, Formaldeyde, and Hydrogen Sulfate) are shown in the chart.